
This newsletter is produced by Teva Pharmaceuticals and the patient pictured above has been compensated for the use of their image.
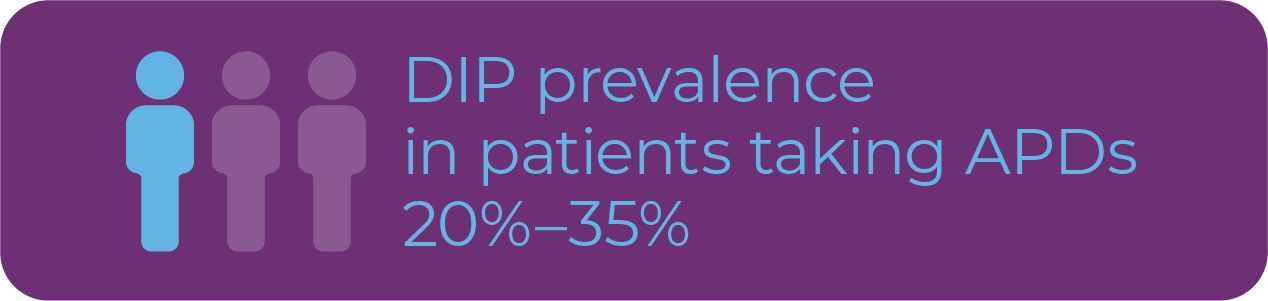
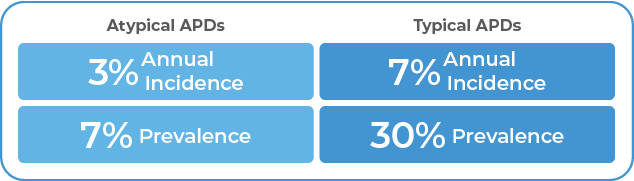
 What Are Extrapyramidal Symptoms? EPS has been commonly used as a general term to describe antipsychotic-induced movement disorders including TD, drug-induced parkinsonism (DIP), akathisia, and dystonia.8 However, in the clinical setting, EPS commonly refers to DIP.8 DIP has a prevalence of
20% to 35% in patients taking APDs.9
What Are Extrapyramidal Symptoms? EPS has been commonly used as a general term to describe antipsychotic-induced movement disorders including TD, drug-induced parkinsonism (DIP), akathisia, and dystonia.8 However, in the clinical setting, EPS commonly refers to DIP.8 DIP has a prevalence of
20% to 35% in patients taking APDs.9

References
1. Caroff SN, Campbell EC, Carroll B. Pharmacological treatment of tardive dyskinesia: recent developments. Expert Rev Neurother. 2017;17(9):871-881. 2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of
patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56. 3. Hirschfeld RM, Bowden CL, Gitlin MJ, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with bipolar disorder, second edition. Accessed January 29,
2020. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf 4. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric
Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with major depressive disorder, third edition. Accessed January 29, 2020. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf 5. Avalos DJ, Sarosiek I, Loganathan P, McCallum RW. Diabetic gastroparesis: current challenges and future prospects. Clin Exp Gastroenterol. 2018;11:347-363. 6. Kim J, MacMaster E, Schwartz TL.
Tardive dyskinesia in patients treated with atypical antipsychotics: case series and brief review of etiologic and treatment considerations. Drugs Context. 2014;3:212259. 7. Caroff SN, Miller DD, Dhopesh V, Campbell EC. Is there a rational management strategy for tardive dyskinesia? Decisions should be based on the
course of TD and effective control of psychotic symptoms. Curr Psychiatry. 2011;10(10):22-32. 8. Frei K, Truong DD, Fahn S, Jankovic J, Hauser RA. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16. 9. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs.
tardive dyskinesia—key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2):233-248. 10. van Harten PN, Bakker PR, Mentzel C, Tijssen MA, Tenback DE. Movement disorders and psychosis, a complex marriage. Front Psychiatry. 2015;5:190. 11. Ruhé HG, Mason NS, Schene AH.
Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331-359. 12. Shin JH, Kim D, Jung MW. Differential coding of reward and movement information in the dorsomedial striatal direct and indirect pathways. Nat
Commun. 2018;9(1):404. 13. Zai CC, Maes MS, Tiwari AK, Zai GC, Remington G, Kennedy JL. Genetics of tardive dyskinesia: promising leads and ways forward. J Neurol Sci. 2018;389:28-34. 14. Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci.
2017;18(8):1689. 15. Settell ML, Testini P, Cho S, et al. Functional circuitry effect of ventral tegmental area deep brain stimulation: imaging and neurochemical evidence of mesocortical and mesolimbic pathway modulation. Front Neurosci. 2017;11:104. 16. Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine
interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16(3):137-162. 17. Howe M, Ridouh I, Allegra Mascaro AL, Larios A, Azcorra M, Dombeck DA. Coordination of rapid cholinergic and dopaminergic signaling in striatum during spontaneous movement. Elife. 2019:8:e44903.
18. Gobira PH, Ropke J, Aguiar DC, Crippa JA, Moreira FA. Animal models for predicting the efficacy and side effects of antipsychotic drugs. Braz J Psychiatry. 2013;35(suppl 2):S132-S139. 19. Hagino Y, Kasai S, Fujita M, et al. Involvement of cholinergic system in hyperactivity in dopamine-deficient mice.
Neuropsychopharmacology. 2015;40(5):1141-1150. 20. Chouinard G, Samaha AN, Chouinard VA, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom. 2017;86(4):189-219. 21. Waln O, Jankovic J. An update on tardive dyskinesia: from
phenomenology to treatment. Tremor Other Hyperkinet Mov (NY). 2013;3. pii: tre-03-161-4138-1. 22. Bhidayasiri R, Fahn S, Weiner WJ, Gronseth GS, Sullivan KL, Zesiewicz TA; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the
American Academy of Neurology. Neurology. 2013;81(5):463-469.



This newsletter is produced by Teva Pharmaceuticals and the patient pictured above has been compensated for the use of their image.
.png)
This newsletter is produced by Teva Pharmaceuticals and the patients pictured above have been compensated for the use of their footage.




.png)
- Baseline AIMS total scores were similar between AUSTEDO and placebo - AUSTEDO 9.7 (SD, 4.1)- Placebo 9.6 (SD, 3.8)
- AUSTEDO significantly reduced AIMS total score by 3.0 points (vs 1.6 in the placebo group) at Week 12, (P=0.019, for a treatment effect of −1.4 points)
- Mean AUSTEDO dose was 38.8 mg/day at the end of titration
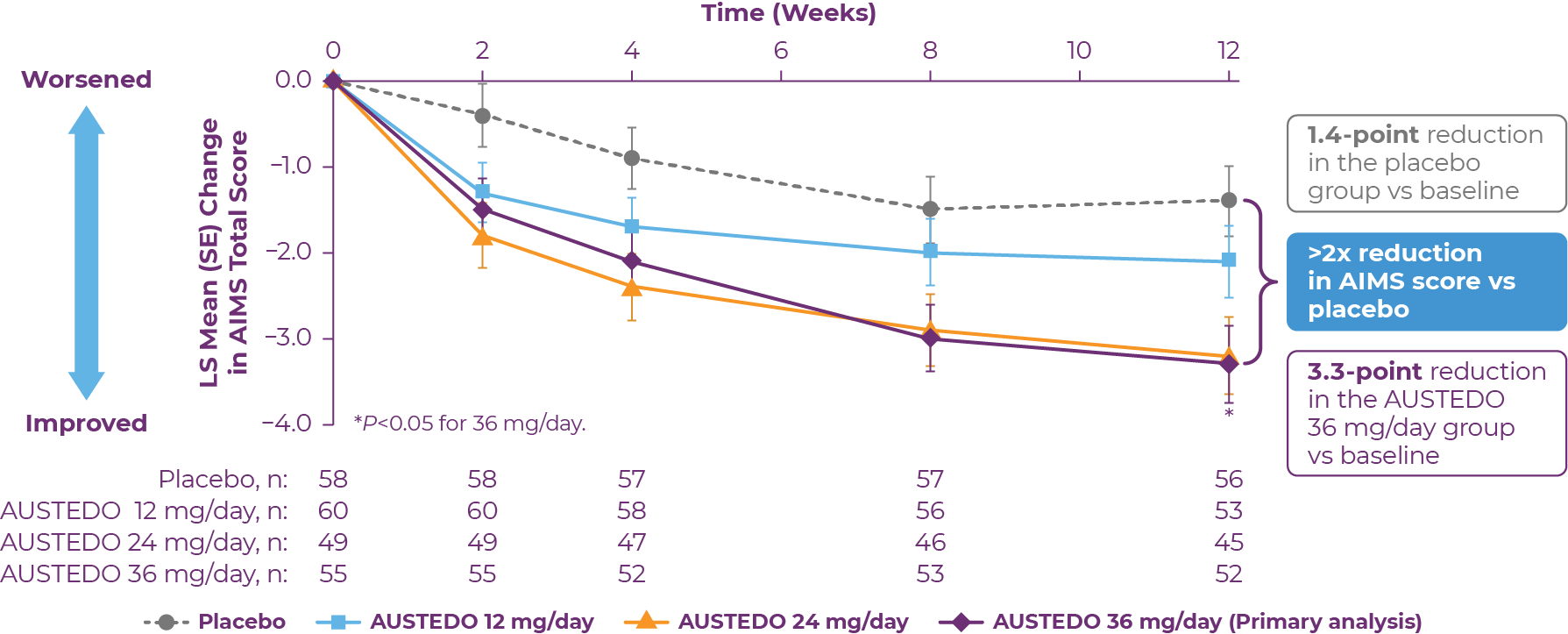 Patients in the AIM-TD study received the AUSTEDO BID formulation.
Safety Results in Clinical Trials Across both pivotal trials, 4% of patients required dose reduction of AUSTEDO due to adverse events vs 2% of patients taking placebo.9 At baseline, 56% of patients treated with AUSTEDO across both studies were taking concomitant antidepressant therapy.20
Patients in the AIM-TD study received the AUSTEDO BID formulation.
Safety Results in Clinical Trials Across both pivotal trials, 4% of patients required dose reduction of AUSTEDO due to adverse events vs 2% of patients taking placebo.9 At baseline, 56% of patients treated with AUSTEDO across both studies were taking concomitant antidepressant therapy.20
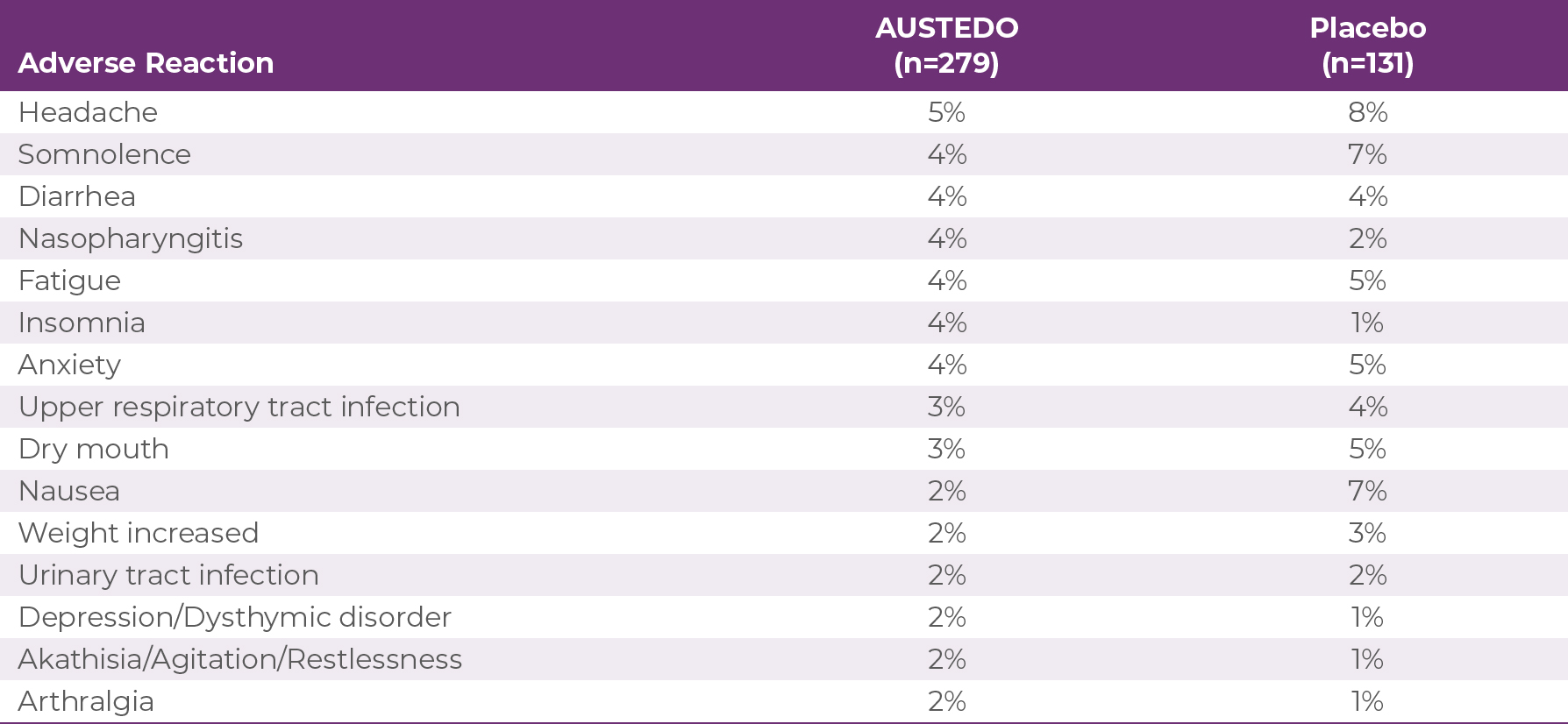
Adverse reactions with AUSTEDO XR are expected to be similar to AUSTEDO.
In patients with TD, AUSTEDO demonstrated significant efficacy compared with placebo in reducing AIMS total score. In controlled clinical studies in patients with TD, the most common adverse reactions were nasopharyngitis and insomnia. AUSTEDO XR is a viable treatment option that allows for the continuation of antipsychotic treatment while concurrently treating the patient's TD.9,18,191. Loughlin AM, Lin N, Abler V, Carroll B. Tardive dyskinesia among patients using antipsychotic medications in customary clinical care in the United States. PLoS One. 2019;14(6):e0216044. 2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2)(suppl):1-56. 3. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder, second edition. Accessed February 11, 2020. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf 4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder, third edition. Accessed February 11, 2020. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf 5. Kim J, Macmaster E, Schwartz TL. Tardive dyskinesia in patients treated with atypical antipsychotics: case series and brief review of etiologic and treatment considerations. Drugs Context. 2014;3:212259. 6. Caroff SN, Miller DD, Dhopesh V, Campbell EC. Is there a rational management strategy for tardive dyskinesia? Decisions should be based on the course of TD and effective control of psychotic symptoms. Curr Psychiatry. 2011;10(10):22-32. 7. Caroff SN. Overcoming barriers to effective management of tardive dyskinesia. Neuropsychiatr Dis Treat. 2019;15:785-794. 8. McEvoy J, Carroll B, Gandhi S, et al. Effect of tardive dyskinesia on quality of life: patient-reported symptom severity is associated with deficits in physical, mental, and social functioning (143). Neurology. 2018;90(15)(suppl):89. 9. AUSTEDO® XR (deutetrabenazine) extended-release tablets/AUSTEDO® current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc. 10. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24. 11. Dean M, Sung VW. Review of deutetrabenazine: a novel treatment for chorea associated with Huntington's disease. Drug Des Devel Ther. 2018;12:313-319. 12. Cepeda C, Murphy KP, Parent M, Levine MS. The role of dopamine in Huntington's disease. Prog Brain Res. 2014;211:235-254. 13. Lohr KM, Masoud ST, Salahpour A, Miller GW. Membrane transporters as mediators of synaptic dopamine dynamics: implications for disease. Eur J Neurosci. 2017;45(1):20-33. 14. Patri M. Synaptic Transmission and Amino Acid Neurotransmitters (chapter). IntechOpen. October 23, 2019. 15. Marsden CD. Dopamine and basal ganglia disorders in humans. Semin Neurosci. 1992;4(2):171-178. 16. Niemann N, Jankovic J. Treatment of tardive dyskinesia: a general overview with focus on the vesicular monoamine transporter 2 inhibitors. Drugs. 2018;78(5):525-541. 17. Stahl SM. Mechanism of action of vesicular monoamine transporter 2 (VMAT2) inhibitors in tardive dyskinesia: reducing dopamine leads to less "go" and more "stop" from the motor striatum for robust therapeutic effects. CNS Spectr. 2018;23(1):1-6. 18. Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003-2010. 19. Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595-604. 20. Data on file. Teva Neuroscience, Inc.



IMPORTANT SAFETY INFORMATION
Indications and Usage
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are indicated in adults for the treatment of chorea associated with Huntington's disease and for the treatment of tardive dyskinesia.
Important Safety Information
Depression and Suicidality in Patients with Huntington's Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington's disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington's Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington's disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington's disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see accompanying full Prescribing Information, including Boxed Warning.





New York Medical College
Valhalla, New York
Q: Why is it important to accurately differentiate between tardive dyskinesia, or TD, and drug-induced Parkinsonism, or DIP?
A: The most important reason to differentiate between the two is that TD and DIP are treated differently—vesicular monoamine transporter 2, or VMAT2, inhibitor for TD and benztropine or an anticholinergic agent for DIP—and treatment for one can exacerbate the other.1 In addition, their prognoses are different—DIP will eventually resolve after discontinuation of the antipsychotic, which is not the case for TD.1
AUSTEDO XR may cause parkinsonism in patients with Huntington's disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR dose should be reduced; some patients may require discontinuation of therapy.
Q: How can a clinician differentiate between TD and DIP?
A: There are a few simple ways to differentiate TD from DIP. First, carefully observe the movements—DIP movements are rhythmic while TD movements are choreoathetotic and irregular. Second, because DIP is frequently accompanied by rigidity,1 it is helpful to ask the patient to walk a few feet. If he/she demonstrates diminished arm swing, it is likely DIP.2 Also, TD and DIP have different onsets, with DIP typically occurring hours to weeks after antipsychotic initiation or dose increase and TD occurring months to years after antipsychotic initiation.1 In addition, if treatment with an anticholinergic agent improves symptoms, you can assume that it is DIP; if it worsens symptoms, it may be TD.1 Lastly, increasing the dose of the antipsychotic may temporarily improve TD but worsen DIP, while decreasing the dose will do the opposite.1
Q: How has benztropine historically been utilized in Psychiatry?
A: In some cases, psychiatrists have used benztropine prophylactically in order to prevent DIP; however, long-term use is not advisable because it can interfere with cognition, especially memory.1
Q: In which situations would you refer a patient to a movement disorder specialist?
A: Let's consider an older patient, say over 60 years of age, who has been on an antipsychotic for many years with longstanding TD. A recent onset of rhythmic movements is suggestive of Parkinson's disease, or PD, rather than DIP if there has been no major change in the antipsychotic dose or if the patient is on a second-generation antipsychotic and if these symptoms continue upon a decrease in the dose or cessation of the antipsychotic drug.3 This would warrant a referral to a movement disorder specialist and, potentially, a DaTscan to rule out PD or a related condition involving neurodegeneration of the nigrostriatal neurons.4
Q: What is the role of AUSTEDO XR in the treatment of TD?
A: AUSTEDO XR, a VMAT2 inhibitor, is efficacious in the treatment of TD in adults.5 It is a viable treatment option that allows for the continuation of antipsychotic treatment while concurrently treating the patient's TD.5
1. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia—key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2):233-248. 2. Manage drug-induced parkinsonism through early recognition of the condition and discontinuation of the causative agent. Drugs Ther Perspect. 2012;28(12):20-23. 3. Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012;8(1):15-21. 4. Cummings JL, Fine MJ, Grachev ID, et al. Effective and efficient diagnosis of parkinsonism: the role of dopamine transporter SPECT imaging with ioflupane I-123 injection (DaTscan™). Am J Manag Care. 2014;20(5 suppl):S97-S109. 5. AUSTEDO® XR (deutetrabenazine) extended-release tablets/AUSTEDO® current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc.
This newsletter is produced by Teva Pharmaceuticals and Dr Citrome was compensated by Teva for his insights.